For life science graduates, career options after completing a degree in pharmacy, biotechnology, or biochemistry often come down to two promising fields — Quality Assurance (QA) and Regulatory Affairs (RA). Both play vital roles in the pharmaceutical and healthcare industries, ensuring that products are safe, effective, and compliant with national and international standards.
While they share common goals, QA and RA differ in function, responsibility, and career progression. Understanding these distinctions helps students make informed decisions and choose the right pathway for long-term success.
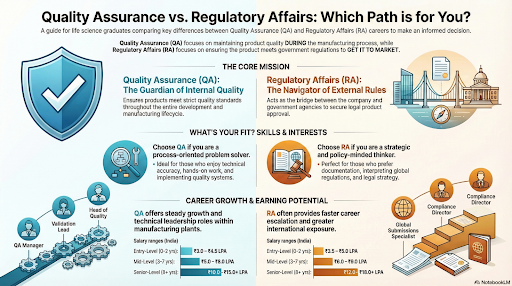
Quality Assurance (QA) focuses on maintaining the standard of pharmaceutical and life science products throughout their development and manufacturing lifecycle. The QA department ensures that every step — from raw material procurement to packaging and distribution — meets strict compliance and safety norms.
Takeaway: QA ensures that products meet regulatory expectations before they ever reach the market.
FAQ — Which is better, QA or RA?
Both QA and RA are essential pillars of the pharmaceutical ecosystem. QA focuses on
maintaining quality standards during production, while RA ensures those standards meet
government regulations.
Regulatory Affairs (RA) is a specialised domain that ensures that healthcare products — including drugs, biologics, and medical devices — comply with global regulations. Professionals in RA act as intermediaries between pharmaceutical companies and government agencies such as CDSCO, USFDA, or EMA.
Takeaway: RA professionals help organisations bring products to market legally and ethically by navigating complex regulations.
FAQ — Can a fresher go directly into RA?
Yes. Fresh graduates can enter the RA field through entry-level positions or by
completing certifications like a drug
regulatory affairs certificate course or a post graduate diploma in drug
regulatory affairs that provide foundational knowledge in compliance and documentation.
Although QA and RA work closely, their focus and scope vary significantly.
| Aspect | Quality Assurance (QA) | Regulatory Affairs (RA) |
|---|---|---|
| Core Focus | Ensuring product quality and process compliance | Ensuring legal and regulatory approval of products |
| Primary Responsibility | Implementing quality systems and audits | Managing submissions, approvals, and compliance with authorities |
| Work Environment | Manufacturing units, QC labs, production floors | Corporate offices, documentation hubs, and regulatory agencies |
| Skill Orientation | Process and product-oriented | Policy, documentation, and legal-oriented |
| Job Outcome | Quality certification and internal compliance | Product approval and external regulatory compliance |
Takeaway: QA maintains product integrity; RA enables product approval and distribution — both are indispensable to pharma operations.
Choosing between QA and RA depends on your interest and professional goals.
| Interest Area | Recommended Career |
|---|---|
| Process control, audits, GMP implementation | Quality Assurance (QA) |
| Regulatory documentation, product approvals, compliance strategy | Regulatory Affairs (RA) |
FAQ — Which has more growth: QA or RA?
Both fields offer steady growth, but RA generally provides faster career escalation and
international exposure, as it involves regulatory communication across countries.
Takeaway: For global career opportunities, RA may offer a broader scope; for plant-level leadership and technical expertise, QA remains a rewarding choice.
Whether you pursue QA or RA, employers look for precision, compliance awareness, and problem-solving abilities.
Takeaway: QA builds internal consistency; RA ensures external compliance — both demand discipline, patience, and continual learning.
Both QA and RA offer promising salary trajectories depending on expertise, certifications, and global exposure.
| Level | QA – Average Annual Salary (India) | RA – Average Annual Salary (India) |
|---|---|---|
| Entry-Level (0–2 yrs) | ₹3.0 – ₹4.5 LPA | ₹3.5 – ₹5.0 LPA |
| Mid-Level (3–7 yrs) | ₹5.0 – ₹8.0 LPA | ₹6.0 – ₹9.0 LPA |
| Senior-Level (8–12 yrs) | ₹10.0 – ₹15.0 LPA | ₹12.0 – ₹18.0 LPA |
| Global/Managerial Roles | ₹18.0 LPA and above | ₹20.0 LPA and above |
FAQ — Is regulatory affairs a high-paying job?
Yes. Regulatory Affairs professionals often earn higher packages due to their critical
role in product approval, global submissions, and compliance management.
Takeaway: While QA offers stability and strong industrial grounding, RA can lead to faster global exposure and higher salaries.
In the evolving world of pharmaceuticals and life sciences, Quality Assurance (QA) and Regulatory Affairs (RA) are equally vital for ensuring product safety and compliance.
Students aspiring to enter these fields can strengthen their qualifications by pursuing a drug regulatory affairs course online, an advanced diploma in drug regulatory affairs, or a PG Diploma in Drug Regulatory Affairs (Jamia Hamdard) — programs that open doors to regulatory roles globally.
For those more inclined toward production and quality control, pursuing specialised training in QA systems and documentation can ensure a secure and rewarding career.
Final Takeaway: QA ensures the quality within, while RA ensures the approval beyond. Together, they define the success of every pharmaceutical product in the market.
Our counselling team is ready to assist you at every step — click the button below to get started.
Need advice? Our experts are on WhatsApp 24/7—message us anytime!