For life science graduates, the pharmaceutical industry offers diverse and rewarding career paths. Among the most sought-after are Pharmacovigilance (PV) and Regulatory Affairs (RA) — two fields that ensure drug safety and compliance throughout a product’s lifecycle.
Both are essential to the success of pharmaceutical and biotech organisations, but they differ in scope, responsibilities, and long-term career growth. Understanding these distinctions can help students and professionals make informed choices based on their interests, skills, and goals.
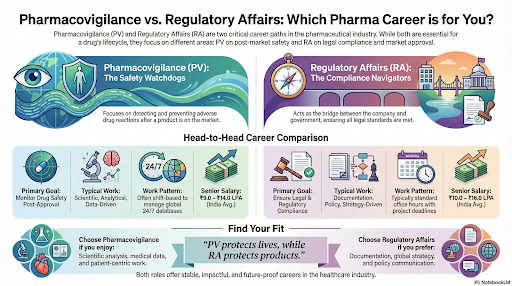
Pharmacovigilance (PV) refers to the science and activities related to detecting, assessing, understanding, and preventing adverse effects or any other drug-related problems. In simple terms, PV ensures that medicines remain safe and effective after they enter the market.
Takeaway: Pharmacovigilance professionals play a vital role in maintaining patient safety and upholding public trust in healthcare systems.
FAQ — Is PV stressful?
Pharmacovigilance can be fast-paced and demanding due to time-sensitive reporting and
global compliance requirements. However, it also offers structured work processes,
strong job stability, and opportunities to specialise in safety analytics or risk
assessment.
Regulatory Affairs (RA) focuses on ensuring that all pharmaceutical and healthcare products comply with national and international regulatory requirements. RA professionals act as the link between pharmaceutical companies and government authorities.
They ensure that drugs, biologics, and medical devices are developed, manufactured, and marketed according to prescribed standards.
Takeaway: The role of the drug regulatory affairs department is critical in guiding a product from research to market while ensuring that every compliance requirement is met.
FAQ — What qualifications are needed for both?
Both careers typically require a degree in pharmacy, life sciences, or biotechnology.
Candidates pursuing RA often benefit from certifications like a drug regulatory affairs
certificate course or a post-graduate diploma in drug regulatory affairs, while PV
aspirants can specialise through pharmacovigilance training programs.
| Aspect | Pharmacovigilance (PV) | Regulatory Affairs (RA) |
|---|---|---|
| Primary Objective | Monitor drug safety after approval | Ensure legal and regulatory compliance of products |
| Work Type | Scientific and analytical | Documentation and policy-driven |
| Key Activities | ADR collection, analysis, signal detection | Dossier submission, product approval, compliance tracking |
| Interaction Level | Collaboration with clinicians, hospitals, and CROs | Coordination with regulatory agencies and company departments |
| Outcome | Ensures post-market safety | Enables product launch and continuity |
Takeaway: PV professionals protect patient safety, while RA professionals protect product legality and company credibility.
Both fields require precision and dedication, but their day-to-day pressures differ.
Pharmacovigilance often involves shift-based roles, particularly in global companies that manage 24-hour safety databases. Timely reporting of adverse events is mandatory, which can occasionally lead to workload peaks.
Regulatory Affairs, on the other hand, follows project timelines around product submissions and renewals, offering a relatively consistent schedule.
| Parameter | Pharmacovigilance | Regulatory Affairs |
|---|---|---|
| Work Pattern | Shift-based (global coordination) | Standard office hours |
| Stress Level | Moderate to high (deadlines) | Moderate (document reviews) |
| Flexibility | Limited | Higher |
FAQ — Can PV professionals transition to RA?
Yes. Many PV professionals move into Regulatory Affairs after gaining 2–3 years of
experience. Their understanding of safety data, documentation, and compliance makes them
strong candidates for RA roles.
Both PV and RA offer attractive salaries and career advancement, but RA often leads to faster international exposure due to its global regulatory nature.
| Career Stage | Pharmacovigilance (Avg Annual Salary in India) | Regulatory Affairs (Avg Annual Salary in India) |
|---|---|---|
| Entry-Level (0–2 yrs) | ₹3.0 – ₹4.5 LPA | ₹3.5 – ₹5.0 LPA |
| Mid-Level (3–7 yrs) | ₹5.0 – ₹8.0 LPA | ₹6.0 – ₹9.0 LPA |
| Senior-Level (8–12 yrs) | ₹9.0 – ₹14.0 LPA | ₹10.0 – ₹16.0 LPA |
| International Roles | ₹20.0 LPA and above | ₹22.0 LPA and above |
Takeaway: While both roles are in demand worldwide, RA professionals often engage directly with international authorities, offering broader exposure and higher salary ceilings.
FAQ — Which job is better: Pharmacovigilance or Regulatory Affairs?
If you enjoy scientific analysis and medical data, PV may suit you better. If you prefer
documentation, communication, and strategy, RA offers a more structured and global
career path.
When deciding between Pharmacovigilance and Regulatory Affairs, consider these factors:
| Interest Area | Recommended Career Path |
|---|---|
| Drug safety, data analysis, and medical writing | Pharmacovigilance |
| Compliance, documentation, international policy | Regulatory Affairs |
| Desire for patient-centric work | Pharmacovigilance |
| Interest in product approvals and global strategy | Regulatory Affairs |
Takeaway: Pharmacovigilance builds expertise in patient safety, while Regulatory Affairs provides a gateway to global compliance leadership. Both offer stable and meaningful careers in healthcare.
In the pharmaceutical world, Pharmacovigilance ensures the continued safety of drugs post-launch, while Regulatory Affairs ensures compliance and product approval across markets. Together, they form the backbone of drug development, safety, and distribution.
Life science graduates who wish to pursue careers in compliance, policy, or documentation can enhance their prospects by enrolling in specialised courses like an advanced diploma in drug regulatory affairs, a PG Diploma in Drug Regulatory Affairs (Jamia Hamdard) , or a drug regulatory affairs course online.
Students looking for affordable options can explore DRA course fees in Delhi to find certification programs that match their goals.
Final Takeaway: Pharmacovigilance protects lives; Regulatory Affairs protects products — and both create impactful, future-proof careers for life science graduates.
Our counselling team is ready to assist you at every step — click the button below to get started.
Need advice? Our experts are on WhatsApp 24/7—message us anytime!